CASE REPORT | https://doi.org/10.5005/jp-journals-10045-00161 |
Management of AKI in Rhino-sinosal Mucormycosis Treated with Amphotericin B
1,3,4Department of Nephrology, RajaRajeswari Medical College and Hospital, Bengaluru, Karnataka, India
2Department of General Medicine, RajaRajeswari Medical College and Hospital, Bengaluru, Karnataka, India
Corresponding Author: MVS Shreyas, Department of Nephrology, RajaRajeswari Medical College and Hospital, Bengaluru, Karnataka, India, Phone: +91 9342550200, e-mail: shreyasmalepati@gmail.com
How to cite this article Prasad SM, Shreyas MVS, Prakash MS, et al. Management of AKI in Rhino-sinosal Mucormycosis Treated with Amphotericin B. J Med Sci 2020;6(4):70–73.
Source of support: Nil
Conflict of interest: None
ABSTRACT
We describe a case of rhino-sinosal mucormycosis in a CAT-B coronavirus disease-2019 (COVID-19) patient who developed an AKI, 2 weeks following her recovery from COVID-19. The case report explores the impact of appropriate fluid balance on the treatment of AKI in a patient receiving Inj amphotericin B for the management of rhino-sinosal mucormycosis which she contracted during her COVID-19 infection.
Keywords: COVID-19, Fungal infection, Hypervolemia.
Introduction
Kidney involvement in patients with coronavirus disease-2019 (COVID-19) is common and can range from the presence of proteinuria and hematuria to AKI requiring RRT.1
Invasive fungal infections frequently occur in immunocompromised patients and critically ill patients and are associated with high rates of morbidity and mortality.2
The use of amphotericin B is critical in treating invasive fungal infections, despite its potential to cause nephrotoxicity.
The complex interactions involved in treating mucormycosis with Inj amphotericin B in a patient who has a preexisting AKI post-COVID-19 infection, are dealt with, in this case report.
The report also intends to shed light on the conundrum of “the relationship between AKI and fluid overload as a cause or consequence”.3
CASE DESCRIPTION
58/F, a known case of T2DM, HTN, contracted COVID-19 and was being treated for the same with systemic antibiotics, steroids, and remdesivir along with oxygen support. A week after the hospitalization, she developed pain over the left side of the face and left nasal stuffiness which upon MRI brain with PNS revealed mucosal thickening and few hypointense areas and heterogeneous enhancement in left maxillary and ethmoidal sinus? Fungal etiology (Fig. 1).
FESS-modified Denker approach (Fig. 2) with histopathological examination of the specimens and KOH mount concluded the diagnosis of mucormycosis (Fig. 3).
She was being treated with oral posaconazole 300 mg OD I/V/O and unavailability of Inj amphotericin B. Her renal parameters at the time of diagnosis and the following week of treatment were normal with a baseline creatinine of 0.8 mg/dL, USG abdomen showed normal-sized kidneys with well-maintained corticomedullary differentiation. She was discharged with a regular follow-up regimen after 4 weeks of hospitalization.
Two weeks after the discharge, she was readmitted with complaints of loosening of left upper molar teeth with puffiness of her face and swelling of bilateral lower limbs. MRI brain showed “mild mucosal thickening of left ethmoidal and maxillary sinus extending to the floor of the orbit, premaxillary, infratemporal fossa. Tiny erosions over alveolar margins of maxilla left side with marrow edema noted” suggestive of extension of mucormycotic growth. Further evaluation revealed deranged renal parameters suggestive of an AKI stage 3 [B. urea-52 mg/dL, S. creatinine-3.2 mg/dL] and severe hypervolemic hyponatremia [Na+ 114 mEq/L]. USG KUB was unremarkable. The urine routine was normal (Table 1).
The nephrologist advised fluid restriction, IV furosemide, hyponatremia correction with 3% NaCl and antibiotics coverage with other supportive measures to be given along with initiation of Inj amphotericin B-lipid complex at a dose of 5 mg/kg/day. Discontinued Telmisartan.
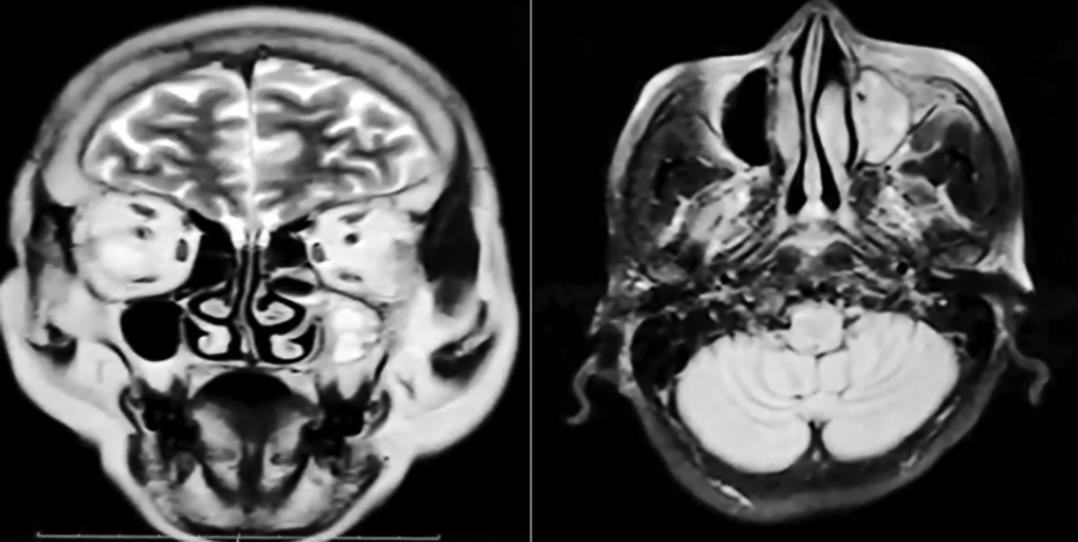
Fig. 1: Before FESS–coronal and axial T2-weighted sequence showing mucosal thickening and few hypointense areas and heterogeneous enhancement in left maxillary and ethmoidal sinus

Fig. 2: After FESS–T2 and FLAIR hyperintensities are noted along the left premaxillary and infraorbital regions. Post-op changes of FESS are noted
The patient required aggressive therapy with Inj amphotericin B despite deranged renal functions. As her hypervolemia was treated, her creatinine was reduced and hyponatremia resolved; however, she developed hypokalemia (K+ 3.1) (multifactorial-furosemide, amphotericin, insulin therapy, poor intake); furosemide dose was reduced; she was initiated on potassium supplementation (oral + IV) and was corrected with a dose of 200 mEq/day for initial 2 days and required maintenance dose of oral KCL of about 60–100 mEq/day during her ICU stay.
After 7 days of re-admission, revision FESS with dentoalveolar resection and primary closure was performed subsequently, when her acute derangements resolved (creatinine reduced to 1.3 mg/dL from 3.2 mg/dL).
The patient was hydrated well before and after administration of amphotericin B according to protocol. Consequently, worsened hypervolemia. On the POD 6 of revision FESS, she developed spikes of fever (2 episodes) and her counts rose from <7000 to 27,000 with the elevation of serum creatinine from 1.3 to 1.9 within a day. Serum procalcitonin was 0.88 ng/mL (Table 1). Urine output dropped to a meager 600 mL. USG abdomen did not reveal any infective foci. A nephrology review was sought again, opined fluid restriction, re-initiated IV diuretics, and continuation of amphotericin B.
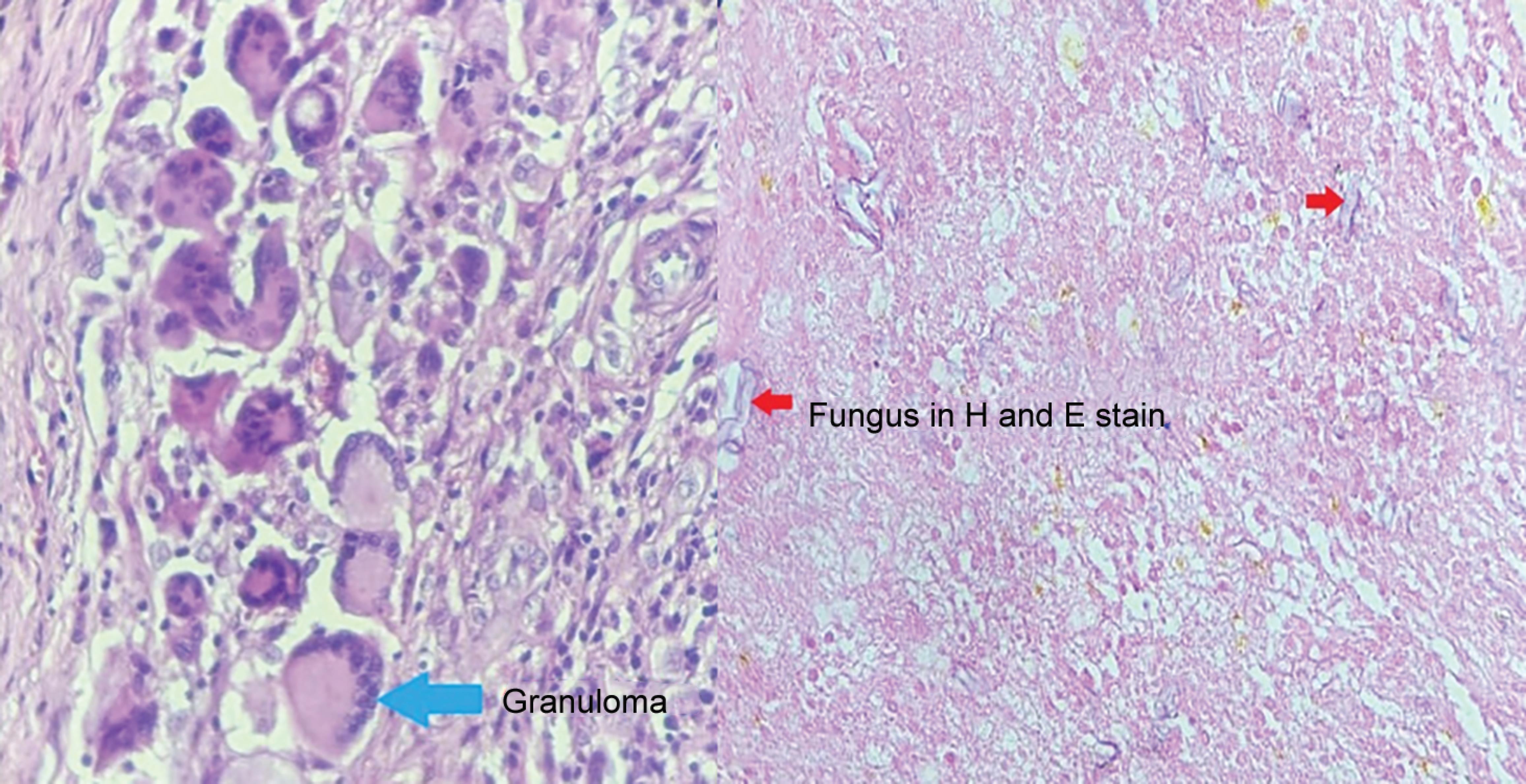
Fig. 3: Histopathology—H and E section studied shows multiple granulomas and mucor fungal species
| Investigation | Presentation day 0 | Transient recovery day 8 | Worsening day 14 | Recovery day 18 | Discharge day 30 |
|---|---|---|---|---|---|
| Hb | 8.3 | 9.0 | 8.0 | 9.8 | 10.4 |
| TLC | 11.78 | 6.79 | 26.8 | 8.0 | 6.0 |
| N/L | 83/11 | 80/12 | 95/2 | 84/12 | 76/20 |
| Platelets | 5.92 | 3.02 | 0.67 | 0.68 | 4.0 |
| Urea | 52 | 40 | 56 | 60 | 21 |
| Creatinine | 3.2 | 1.3 | 1.9 | 1.3 | 1.1 |
| TB | 0.5 | 0.5 | 0.8 | 0.8 | 0.8 |
| ALT | 8 | 9 | 37 | 17 | 12 |
| AST | 15 | 16 | 99 | 16 | 16 |
| ALP | 97 | 76 | 146 | 236 | 208 |
| pH | 7.4 | 7.38 | 7.43 | 7.28 | 7.45 |
| pCO2 | 26 | 34 | 33 | 45 | 26 |
| HCO3− | 17 | 20 | 23 | 20 | 20 |
| pO2 | 84 | 90 | 90 | 97 | 100 |
Suspecting an infected right IJV central line, cultures from the blood drawn from it and its tip were sent and an alternate central line was inserted. Foley’s catheter was replaced. On day 16 of admission, she was diagnosed to have hospital-acquired pneumonia. Sputum cultures revealed Klebsiella pneumoniae, prompting antibiotic modifications, while blood cultures revealed no growth.
She gradually improved, as evidenced by falling total counts and decreasing serum creatinine and good urine output. Her creatinine has stabilized around 1.1 despite the continuation of Inj amphotericin, which was later discontinued after she received a cumulative dose of 5 g over 3 weeks, and thereafter she was switched over to oral posaconazole. She was discharged after 30 days of re-admission with the final diagnosis of post-COVID-19-rhino-sinosal mucormycosis s/p revision FESS, comorbidities-T2DM, hypertension, AKI–multifactorial–resolved, hypervolemic hyponatremia, hypokalemia–multifactorial, hospital-acquired pneumonia, central line-associated bacterial infection.
DISCUSSION
The effective management of COVID-19 lies in the appropriate usage of antivirals and corticosteroids, which unfortunately paves way for secondary infections, especially if the patient is diabetic and is using oxygen therapy from contaminated sources. Secondary infections are common in hospitalized severely ill COVID-19 patients, encompassing between 10 and 30% of cases, fungal being 10 times more common.4 Invasive pulmonary aspergillosis complicating the course of COVID-19 is widely recognized,5 however, mucormycosis is uncommonly suspected or diagnosed.
Mucormycosis, an invasive fungal infection caused by filamentous fungi belonging to the class zygomycetes, order Mucorales, is on the rise among patients suffering from COVID-19. The fatality rate of mucormycosis is 46% globally.4
The spores of the fungi found ubiquitously in the environment may cause infection only in those who are immunocompromised either due to a malignancy or HIV infection–transplantation–steroid therapy.
Depending on the location of involvement, we have six clinical syndromes:
- Rhinocerebral.
- Pulmonary.
- Gastrointestinal.
- Cutaneous.
- Disseminated.
- Miscellaneous.6
Histological evaluation of mucormycosis is the mainstay of diagnosis (Fig. 3). Diagnosis occurs through observing non-septate or minimally septated broad, ribbon-like hyphae (10–20 μm) invading blood vessels.7
Radiological features include a rim of soft tissue thickness along with the paranasal sinuses. Complete sinus opacification, gas fluid levels, and obliteration of nasopharyngeal tissue planes can also occur8 (Fig. 1).
The treatment of mucormycosis involves nephrotoxic agents like amphotericin B, where the liposomal version is said to be less nephrotoxic.
KDIGO guidelines recommend that exposure to nephrotoxic drugs should be limited in patients at risk of developing AKI and that a risk/benefit assessment is carried out to with the risk of developing the AKI against the risk associated with not using the agent.9 When there is a clinical diagnosis of a potentially life-threatening invasive fungal infection, AKI is not a contraindication to starting treatment with lipid-based-amphotericin B formulations.
Compared with conventional amphotericin B, the incidence of nephrotoxicity was significantly reduced with the use of liposomal amphotericin B (14.5 vs 32.5%) or lipid emulsion amphotericin B (12.2 vs 30.6%).10
There was no obvious conventional cause of AKI in our patient at the time of presentation. There is growing evidence that AKI is prevalent among patients with COVID-19, particularly among patients in the ICU.1 The pathophysiology of the development of AKI in COVID-19 has been described under direct and indirect mechanisms.1 Direct mechanisms [viral cytopathic effects] (1) Coagulopathy (2) Endothelial dysfunction (3) Inflammation (4) Complement activation. Indirect mechanisms (1) Fluid mismanagement (2) Mechanical ventilation (3) Nephrotoxins (4) Organ crosstalk (5) Fever/sepsis/hypovolemia.
Patients with AKI who are taking amphotericin B, which has the potential to affect renal tubular function should receive high-quality supportive care. Fluid balance, electrolytes, and acid-base status must all be managed optimally. Fluid administration, rather than decreased urine production, was found to be independently linked with progression from AKI stage I to stage III in a retrospective analysis.11 We suggested discontinuing hydration pre- and post-amphotericin B and were advised to keep a negative fluid balance of around 1,000 mL/day, which aided the patient to achieve euvolemia and recovery of AKI. She also received potassium supplementation of 60 to 100 mEq/day to maintain eukalemia.
Our patient despite having stage 3 AKI, was managed conservatively without initiation of dialysis. Recent STARRT-AKI trial concluded early initiation of renal-replacement treatment in critically ill patients with stage 2 or 3 AKI did not result in decreased mortality at 90 days than a standard strategy. The standard strategy group did not receive RRT until one or more of the following was present:
- Potassium ≥6 mmol/L.
- pH ≤7.2.
- Bicarbonate ≤12 mmol/L.
- PaO2/FiO2 ≤200 + Volume overload.
- Persistent AKI for 72 hours after randomization.
Our patient developed high-grade fever and shock on day 14 of admission which was supported with noradrenaline and vasopressin. The catheter was immediately replaced; in addition, antibiotics were escalated to Inj. vancomycin and meropenem. In the case of an underlying invasive fungal infection, procalcitonin is helpful to differentiate bacteremia from fungemia. As in our case, procalcitonin was 0.88 ng/mL, which prompted us to treat it as bacterial sepsis. She had an improving course in the ICU thereafter and was discharged with normal creatinine and electrolytes.
CONCLUSION
It is critical to maintaining euvolemia. Because one size does not fit all, hydration before and after amphotericin B is unnecessary in patients who are already volume overloaded. Fluid overload is a risk factor for AKI and the aggravation of preexisting AKI, and it must be avoided as far as possible.
If the benefit outweighs the risk, provide the entire dose of amphotericin B even if the patient’s renal function is impaired. The drug could be life-saving. Don not stop potassium supplementation when the requirement is more than usual or when hypokalemia is corrected. Assessing maintenance supplemental dose of KCL is vital to avoid dyskalemia.
Sudden shock and worsening of clinical condition in an improving ICU patient, one must suspect CLABSI and never hesitate to replace the central line. Early detection and early intervention are the keys.
Because mucormycosis is aggressive and invasive, it necessitates a collaborative effort and multidisciplinary strategy that is both aggressive and intrusive. In such situations, being conservative only implies we have given up.
REFERENCES
1. Nadim MK, Forni LG, Mehta RL, et al. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) workgroup. Nat Rev Nephrol 2020;16(12):747–764. DOI: 10.1038/s41581-020-00356-5.
2. Takazono T, Tashiro M, Ota Y, et al. Factor analysis of acute kidney injury in patients administered liposomal amphotericin B in a real-world clinical setting in Japan. Sci Rep 2020;10(1):15033. DOI: 10.1038/s41598-020-72135-y.
3. Ostermann M, Straaten HM, Forni LG. Fluid overload and acute kidney injury: cause or consequence? Crit Care 2015;19(1):443. DOI: 10.1186/s13054-015-1163-7.
4. Maini A, Tomar G, Khanna D, et al. Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int J Surg Case Rep 2021;82:105957. DOI: 10.1016/j.ijscr.2021.105957.
5. Garg D, Muthu V, Sehgal IS, et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia 2021;186(2):289–298. DOI: 10.1007/s11046-021-00528-2.
6. Gupta KL, Gupta A. Mucormycosis and acute kidney injury. J Nephropathol 2012;1(3):155–159. DOI: 10.5812/nephropathol.8111.
7. Hernández JL, Buckley CJ. Mucormycosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
8. Weerakkody Y. Sinonasal mucormycosis: radiology reference article. Radiopaedia Blog RSS 2016 https://radiopaedia.org/articles/sinonasal-mucormycosis?lang=us . (accessed August 22, 2021).
9. Armstrong-James D, Koh M, Ostermann M, et al. Optimal management of acute kidney injury in critically ill patients with invasive fungal infections being treated with liposomal amphotericin B. BMJ Case Rep 2020;13(5):e233072. DOI: 10.1136/bcr-2019-233072.
10. Mistro S, Maciel Ide M, de Menezes RG, et al. Does lipid emulsion reduce amphotericin B nephrotoxicity? a systematic review and meta-analysis. Clin Infect Dis 2012;54(12):1774–1777. DOI: 10.1093/cid/cis290.
11. Raimundo M, Crichton S, Martin JR, et al. Increased fluid administration after early acute kidney injury is associated with less renal recovery. Shock 2015;44(5):431–437. DOI: 10.1097/SHK.0000000000000453.
________________________
© The Author(s). 2020 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.